“But you don’t look that sick. Did you walk here, or did someone drive you?”
“Your cough isn’t a dry cough, like the virus. It’s a wet cough, like a cold.”
On Mar. 19, Carlos Del Valle, a 65 year-old man claimed that a doctor at the Río Piedras Medical Center refused to run the COVID-19 test on him, despite having a referral from his doctor, who told him he could be at risk because he was showing symptoms and had been exposed to people infected with the virus.
“I’m an old person, I have diabetes and I feel weak,” the man told the doctor, who did not think those circumstances were enough to authorize the test administered by the Health Department.
Del Valle is not alone in his experience. The Center for Investigative Journalism (CPI, in Spanish) has learned of at least nine cases in the last week where the government has denied testing for COVID-19.
Luz Otero’s niece is in the intensive care unit after being admitted for coronavirus last Friday. She’s 30 years-old and works at the Luis Muñoz Marín International Airport.
She began running a fever on Sunday, March 15, so she was unable to complete her shift at an airport store. On Monday, when the social distancing and lockdown order signed by Gov. Wanda Vázquez-Garced was already in effect, her condition worsened. Many doctors had closed their offices, so Otero consulted a doctor, a family friend, who prescribed mycoplasma and influenza tests, knowing that getting the coronavirus test without having those first would be difficult. The results came back Tuesday, both negative.
She had a urine test and a chest X-Ray done, neither of which provided information for a diagnosis. The woman continued to weaken during the week and the doctor finally issued the order for the COVID-19 test, which was done on Friday, March 20 in tents set up for this at the Río Piedras Medical Center.
There, said Luz, she had to wait for more than three hours under the blazing sun for medical staff to take the sample from her niece, even though there were not many people waiting. The young woman was hospitalized that same day and received treatment for pneumonia until confirmation came Sunday that she was infected with COVID-19, a week after she first developed symptoms.
“She wouldn’t have reached the condition she’s in if on that same Tuesday, once she tested negative for influenza, mycoplasma and the CBC, we could have gone to the Río Piedras Medical Center tent to get her tested for COVID-19. I’m sure we wouldn’t have reached this point,” said Otero, frustrated.
“My niece is a healthy girl. She’s 30 years old,” she said with regret.
She told the CPI that one of her niece’s co-workers, who presented symptoms later in the week, was able to get tested quicker, and also tested positive. She is recovering at home.
The time factor, Otero believes, is decisive.
Meanwhile, Chris Cortés told the CPI that it took a second visit to the San Francisco Hospital in Río Piedras for his 80-year-old grandfather to get authorization for a COVID-19 test. A week earlier, on Saturday, the 14th, the man had arrived by ambulance to the same hospital with symptoms of fever and weakness; the results for mycoplasma and influenza tests were negative. No medical personnel suggested then that he be tested for COVID-19. They released him that same Saturday, the 14th.
Upon his return to the hospital a week later, the man was finally declared a suspicious case of COVID-19 and was tested on Monday, the 23rd. Two days later, he tested positive.
Given situations like these, several municipalities around the island have taken the initiative to administer or buy their own tests without depending on coordinating with the Health Department.
Municipalities activate their own initiatives
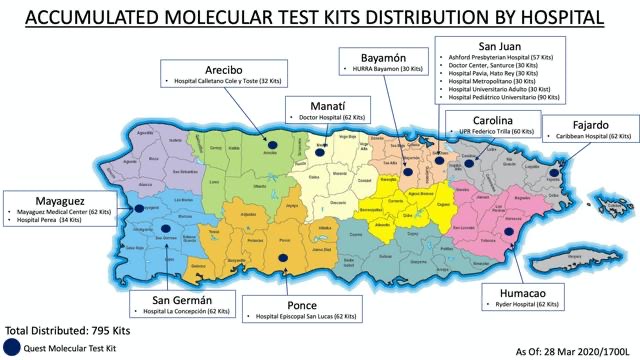
Thirteen municipalities (out of 78) have already announced that they are administering COVID-19 tests in labs and hospitals with whom they have reached agreements. However, the testing process lacks consistency because each municipality has different criteria to establish who can receive the service and how it will be administered.
The towns already providing the service are: San Juan, Bayamón, Caguas, Carolina, Ponce, Mayagüez, Manatí, Fajardo, Naguabo, Canóvanas, Añasco, Cataño and Humacao. Meanwhile, Isabela, Hatillo, Quebradillas and Rincón are currently in negotiations with private labs to buy the tests.
San Juan and Canóvanas began testing during the third week of March. As of this Monday, San Juan had administered 501 tests and Canóvanas, 20, and had results for one and two positives, respectively. The rest of the towns began administering the tests just a few days ago, so they are still waiting for results.
While some municipalities require a visit to a doctor to tend to the patients in person before being tested, for others an evaluation over the phone is enough. Some towns, such as Bayamón, are paying only for the tests for their employees, while others are paying tests for all their residents.
In some cases, drive-up’s have been set up to get tested, and in others, the Diagnostic and Treatment Center (CDT, in Spanish) or municipal health centers, participate in the process. Furthermore, towns like Carolina have delegated the entire process to a private institution. There, the San Fernando Hospital operates as a public-private partnership between the Carolina municipal government and the corporation Doctor’s Center.
The lack of uniformity in the way each town administers the COVID-19 test highlights the absence of a protocol or a national coordinated plan by the Health Department, and raises the question of whether a consistent collection of data is being done on those who are tested. The CPI attempted to reach the Medical Sciences Campus team in charge of data collection on COVID-19 but got no response.
Towns like Naguabo and Fajardo told the CPI that they do not depend on the Department of Health approvals or guidelines. “The State Epidemiologist is consulted, but (the test) it’s done without their OK. We let them know, but they don’t expect it,” the Mayor of Naguabo, Noé Marcano, told the CPI.
The municipal administrator said his decision not to coordinate with the Health Department came from an experience in which the central government agency was slow responding to a request to test one of their patients with symptoms associated with COVID-19.
In the case of Fajardo, the interaction with the Department of Health is limited to reporting. The coordination is done through the Caribbean Medical Center, a private hospital, the Municipal Emergency and Disaster Management Office told the CPI.
In Añasco, Castillo Laboratory administers the tests if the patient comes with a referral from a private family doctor after showing symptoms associated with COVID-19.
That same doctor — not the municipal government — will be the one to contact the Health Department, Añasco Municipal Police Commissioner Lizbeth Cruz told the CPI. In this scenario, the municipality’s role is just to pay for the test done on the Añasco resident at a private lab.
Most city officials who spoke with the CPI said patients with symptoms get mycoplasma and influenza tests first. If they come up negative in those tests, the next step is to authorize the COVID-19 test. In other municipalities, signs of mild and severe symptoms are enough reason for a doctor to authorize the administration of the COVID-19 test.
But this can pose a problem.
“Sometimes mycoplasma-positive doesn’t mean someone doesn’t have coronavirus. This is why a clinical evaluation is important,” Dr. Carlos Mellado, who works at the CDT in Canóvanas, one of the towns that administers the COVID-19 tests to its residents, told the CPI.
“I tested positive for mycoplasma. So, they didn’t want to do the COVID test. I asked for a copy of my results because my internist physician wanted to see my CDC labs and with those results, my doctor told me that I needed to keep insisting on the test because I had something viral,” a 31-year-old woman told Telemundo de Puerto Rico, after the Río Piedras Medical Center denied her the COVID-19 test for having tested positive for mycoplasma. Days later, she was finally tested and the result came back positive for coronavirus.
Other criteria the municipalities depend on to decide who is tested is the outright appearance of those symptoms associated with the virus.
“The resources should be saved for those who have symptoms, from mild to acute. We don’t want people with mild symptoms to take away from someone with more severe symptoms,” said San Juan Mayor Carmen Yulín Cruz-Soto, in an interview with the CPI.
San Juan reported that so far, they have administered 501 tests, of which, one has tested positive and 485 are awaiting results. She also said they plan to do about 25 tests per day.
San Juan was the first municipality to announce it would run COVID-19 tests. The residents of Puerto Rico’s capital city may receive the service through a drive-up system located in front of the Río Piedras CDT, as long as they get a medical screening first.
The decision to only administer the test to people with severe symptoms raises the question of how to handle cases of asymptomatic people, which, according to experts, represent the majority and are the main focus of contagion.
Testing missteps by local and federal governments
When the Government of Puerto Rico reported the first possible case of COVID-19 on the island on Sunday, Mar. 8, every hospital and health center in the United States relied exclusively on the test developed by the CDC to confirm or rule out their suspicions of the illness caused by coronavirus.
As of that date, the United States had barely confirmed 541 cases across the country and had done just over 3,000 tests.
The methodology developed at the CDC is based on a molecular technique known as PCR (polymerase chain reaction). It consists of detecting the virus’ RNA (ribonucleic acid) genetic material and replicating it exponentially to identify it. It takes four to six hours to get a result.
“It requires special equipment and that’s why it’s quite complicated and takes a long time to do,” Juan Rexach, president of the Puerto Rico Association of Clinical Laboratories, explained to the CPI.
For its application in the United States, the CDC’s specific methodology has an Emergency Use Authorization from the U.S. Food and Drug Administration (FDA), which makes it possible for “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious diseases or conditions caused when there are no adequate, approved, or available alternatives.”
On Feb. 4, 2020, the U.S. Health Secretary, Alex Azar, determined that the public health emergency justified actions for the rapid detection of COVID-19 cases. This involved speeding up processes for companies and scientific research entities to use their own methodologies for detecting coronavirus without having to go through the agency’s regular process, which is usually long and expensive.
The CDC was the first entity to get emergency use authorization the same day as Azar’s statement. The next day, on Feb. 5, the distribution of kits began so that laboratories in the states and territories could replicate the test without having to send the samples to Atlanta, where the CDC headquarters are located.
However, while validating the lab tests, most came back with inconclusive results, and a few — including those from New York state and New York City, the epicenter of the pandemic — had problems with two of the reagents. A reagent is the chemical or biological material that is used to process a sample and obtain a result.
During the time it took to manufacture new reagents, all samples of possible cases had to be sent to Atlanta, Nancy Messonnier, director of the CDC’s National Center for Immunization and Respiratory Diseases, said on February 12.
It wasn’t until two weeks and two days later that Messonnier came up with a solution to the problem. She said laboratories could continue using the kits discarding the problematic reagents, since it had been concluded that they do not affect the test result. In addition, the CDC ordered the production of more kits to be distributed.
Another two weeks passed and on Mar. 13, Gov. Wanda Vázquez-Garced announced that the Health Department’s Public Health Laboratory would begin conducting the CDC methodology on the island.
Meanwhile, on March 12, the FDA began issuing emergency use authorizations — like the one granted to the CDC’s — to commercial companies to develop their own tests. Swiss Roche Molecular Systems was the first private company authorized to do so. Since then, 17 other companies have gotten emergency use authorization for their PCR methodologies. Among these are laboratory testing giants Quest Diagnostics and LabCorps.
It’s through these two companies that some Puerto Rico municipalities have managed the analysis of samples from their employees or residents. They require nursing or medical technology personnel to insert a long cotton swab through the nose to the throat, where the highest viral load is found.
As Rexach explained, this sample is placed in a tube with a special liquid and sent to the lab for analysis. In the case of the towns that have established agreements with Quest and LabCorps, they must send their samples to those companies’ stateside offices and they will return the results in four to six days.
Federal guidelines contradict international recommendations
The difficulty in getting tests for people who could be infected has been constant since the beginning. During the first two weeks after the first test was sent on March 9, there were days when the Health Department did not submit any samples and others when they sent up to 46, for an average of 36 daily. This figure is 14 less than the minimum capacity of 50 a day that former State Epidemiologist, Carmen Deseda, said the Health Department’s Public Health Laboratory could process.
The new Health Secretary Lorenzo González said he will put a third shift to work at the Health Department’s Public Health Laboratory to boost the analysis capacity to 150 samples daily. In addition, he will activate the dengue branch laboratory, attached to the CDC. If successful, up to 300 samples could be processed daily.
Almost three weeks after the first suspicion of the presence of coronavirus on the island, and while the guidelines on who can be tested have been somewhat eased, they’re still strict and contrary to the recommendations of the World Health Organization (WHO) and the way other countries that seem to have overcome the worst part of the pandemic have handled it.
“Due to the wide scale shortages of laboratory supplies and reagents, we strongly urge public health and healthcare professionals to prioritize COVID-19 testing among three specific groups,” says a joint statement by the Association of State and Territorial Health Officials, the Association of Public Health Laboratories and the Council of State and Territorial Epidemiologists issued on Friday, Mar. 20. Together, these three organizations represent virtually the entire public health infrastructure in the United States.
The three groups suggest focusing the tests on healthcare workers and first responders with COVID-19 symptoms; seniors who present symptoms of COVID-19, especially those living in congregate settings; and, individuals who may have other illnesses that would be treated differently if they were infected with COVID-19 and therefore a physician’s opinion is especially important.
The New York Department of Health, which currently struggles with the exponential increase in cases every day, has recognized the shortage. The health authority went even further by establishing that sick people with mild symptoms will not be screened for coronavirus.
“If you think you have COVID-19 and your illness is mild, you don’t need to see your healthcare provider and you will not be tested. Testing will not change what your provider will tell you to do to improve. They will tell you to stay home so you don’t make others sick,” the latest guide states.
“Unless you are hospitalized and a diagnosis affects your care, you will not be examined. Limiting the tests protects healthcare workers and saves essential medical supplies, such as masks and gloves, which are scarce,” it says.
The CDC’s guides also establish that “not everyone needs to be tested for COVID-19.” On its official website, the federal entity justifies this by saying “Most people have mild illness and are able to recover at home” and that “there is no treatment specifically approved for this virus.”
The CDC leaves it up to state and local health departments, or individual physicians, to decide who should be tested.
Waiting for more than a million rapid tests
Medical Task Force members have reiterated that there is a shortage of medical equipment and materials for testing.
“We’re trying to get tests out of nowhere ,” said obstetrician gynecologist Juan Luis Salgado, a member of that team.
“There are no tests and we’re doing the unspeakable. We’re fighting against the world. It’s not that we don’t want to do the tests, it’s that it’s very difficult to get them.”
On Saturday, the Medical Task Force member and Interim Executive Director of the Comprehensive Cancer Center, Marcia Cruz-Correa, said that the Health Department had ordered the purchase of 1,350,000 tests.
Of those, 1.3 million are the so-called “rapid tests,” or serological tests, which consist of a blood sample to analyze the presence of antibodies to the virus. The Health Department ordered 1 million tests from a company in Australia, and the remaining 300,000 from a Chinese company, Cruz-Correa said.
“These tests are designed for screening. They don’t give you a definitive diagnosis,” Rexach warned.
“They’re not looking for the virus, but the body’s response to the virus, which are the antibodies,” she added. Depending on the type of antibodies detected, it gives an idea about how long a person has had the virus.
Given the emergency situation, the FDA delegated the diagnosis and treatment for the virus to each state and territory. “So, the Governor has the authority, without the FDA’s authorization, to establish what mechanisms will be used in Puerto Rico,” said Rexach.
Furthermore, the test’s sensitivity and specificity — values that represent its reliability — depend on the company that develops it and the initial studies it carried out.
“That’s why the fact that the FDA quality seal isn’t required right now is very important. For now, it’s a grey area, we have to go by what the manufacturer says. So, it’s important that a quality control mechanism be established in our health system,” Rexach added.
The importance of these rapid tests lies in the possibility of establishing a system and case tracking and specifying the daily growth rate of positive cases.
With the data collected so far, Medical Task Force Epidemiologist Juan Carlos Reyes was able to preliminarily establish that cases double every 3.5 days.
The rapid surveillance system should start this Monday or Tuesday, Medical Task Force Director Segundo Rodríguez-Quilinchini said at a press conference last Saturday.
“The team’s goal is that, starting Monday, we’re going to have a rapid test and surveillance system that will allow us to really tell people if the numbers are going to keep growing at a very fast rate or if we’re able to contain it,” Cruz-Correa said in an interview with David Begnaud.
He explained that rapid tests are only for people who have symptoms, since performing it on an infected, but asymptomatic person would yield a false negative. He explained that this test’s sensitivity level is 95%, so that false negatives could only occur in 5% of cases. If symptoms persist for these patients, a molecular test, or PCR, would be recommended to definitively rule out or confirm the presence of COVID-19.
“Due to the epidemic, given the data we have, and given the diagnostic accuracy of the test, if a person tests positive for the serological test, it’s considered a positive. We don’t need to confirm that, “said Cruz-Correa.
La Fortaleza’s Secretary of Public Affairs, Osvaldo Soto, said the Emergency Management Bureau, in coordination with the Health Department, received 1,000 rapid test kits on Saturday, and that another 5,000 were expected on Sunday.
According to information provided by the Medical Task Force, the Health Department ordered 50,000 PCR-type tests to Quest.
Tests are free of charge
On Mar. 11, the Office of the Insurance Commissioner (OCS, in Spanish) released a regulatory document stating “every health service organization and insurer that underwrites commercial medical plans must provide coverage for COVID-19 diagnostic tests, in accordance with a doctor’s criteria and order, and subject to the terms on the deductible or copayment applicable to laboratory coverage in the insured’s medical plan or policy.”
The CPI contacted the OCS, and Deputy Commissioner Rafael Cestero explained that the coverage for the COVID-19 diagnostic tests applies to all of them, including the rapid tests. Cestero pointed out that the approved “Families First Coronavirus Response Act” states that insurers and health services organizations are required to cover the COVID-19 tests.
Puerto Rico’s Patient Advocate, Edna Díaz-de Jesús, also confirmed that “the health care plans are bound to cover the full payment of the COVID-19 tests, to guarantee that the patient can receive the service free of charge.”
In addition, Deputy Patient Advocate, Alexie Lugo-Canales, spoke with the CPI and urged citizens to keep their laboratory receipts and contact their office if they have problems with the payment and want to file a complaint.

New testing options looming, but materials are scarce
Last Monday, the FDA announced a new policy that allows state and territorial public health laboratories to authorize testing in other laboratories without the need for additional federal approval. With this authorization, the Toledo Clinical Laboratory in Arecibo began the tests on Wednesday and has the capacity to process 1,000 per day.
On Friday, Mar. 20, the FDA also granted an emergency use authorization to Cepheid for the use of its rapid PCR methodology (Xpert Xpress SARS-CoV-2 test), which would enable the analysis of the sample in about 45 minutes.
“This company (Cepheid) made a molecular adaptation with the small machines they have to speed it up. It’s very important to bring this test because that machine and that rapid PCR methodology is relatively common in Puerto Rico. Many small community reference labs and hospitals have this machine, so they would be able to process them,” said Juan Rexach, president of the Association of Clinical Laboratories of Puerto Rico.
Both traditional and rapid PCR methodology require that the sample be taken with a long cotton swab that is inserted through the nose and down the throat. As Rexach explained, it’s very uncomfortable for the patient and usually causes sneezing or coughing. So, it is essential that the nursing or medical technology personnel who take it are properly protected to avoid contagion. If the sample is not taken correctly, it could mean a false negative.
As the rapid PCR methodology becomes available in Puerto Rico, the shortage of reagents and materials that make up the kit for traditional PCR tests complicate the possibility of expanding larger-scale diagnoses.
As reported by The New Yorker on Tuesday, one of the largest Q-tip producers in the world, Copan, is located in Italy, a country that has been paralyzed for more than a week due to the rapid spread of the virus.
In a conversation with journalists on Mar. 18, U.S. Department of Defense Joint Staff Surgeon Brig. Gen. Paul Friedrichs said an Air Force C-17 cargo plane was sent to Italy to transport 500,000 Q-tips to Memphis, where they were transferred to a FedEx plane for distribution.
La Fortaleza confirmed that as of this Sunday, March 29, a combined 1,899 tests had been conducted by the Health Department, the Veterans Administration System and private laboratories.





VA clinic and San Juan stop giving test for since Friday Jan 7,2022
I am a Veteran and I was told today that they ate not giving any more testing for veterans at the Clinic in Puerto Rico ,
The nurse told welcome’s to Puerto Rico
I could not believe he said to me you are on your own !