Finding molecular tests that confirm a COVID-19 diagnosis in Puerto Rico is currently quite challenging, and the government restricts who has access to them. One of the reasons is the lack of materials such as reagents, the chemicals necessary to detect the coronavirus.
“All reference labs have a need for reagents,” said Ilia Toledo, president of the Toledo Clinical Laboratory, one of the largest in Puerto Rico.
In addition, the 200 members of the Association of Clinical Laboratories face problems getting swabs for sampling, its President Juan Rexach confirmed.
The standard justification given for the lack of swabs, transport vials, and chemical reagents — all necessary to administer and process these tests — is fierce competition for these supplies throughout the U.S. and around the world.
However, the reason for this shortage on the Island has been largely due to the government of Puerto Rico’s negligent lack of oversight of the administration of these tests until the end of July, despite knowing since at least March 20 that there would be a shortage, when lab representatives warned them in a meeting in La Fortaleza, as three sources confirmed to the Center for Investigative Journalism (CPI, in Spanish).
In addition, the government failed to prioritize molecular tests since the onset of the pandemic, even though they have been the so-called gold standard for detecting the virus.
In its first strategic plan, the Department of Health stated it would only administer just over 17,980 molecular tests during May and June, despite having the capacity to process up to 30,660 samples per month in public laboratories, according to the document delivered to the U.S. Department of Health and Human Services (HHS).
It neither assigned financial resources or equipment to assist the private laboratories that administer them despite the fact that $85 million in federal funds are available to perform testing and tracking systems.
Gov. Wanda Vázquez Garced announced on August 11 that Swiss multinational manufacturer Roche, the main supplier of reagents in Puerto Rico, was going to guarantee its availability to private reference laboratories to process some 11,000 weekly tests. But what the governor promised during her electoral campaign does not represent an increase. The number stays the same. “That is what Roche has been sending us for months,” said Ilia Toledo, president of Toledo Clinical Laboratory.

Photo by Dennis A. Jones | Metro PR
The governor campaigned speaking of an increase in testing that has not occurred.
A week before the announcement, a Roche spokesperson told the CPI that the shortage situation will not improve for the time being. “Demand for diagnostic instruments, tests and associated materials continue to exceed supply as cases [of infections] spike nationally [in the United States], and we will likely not be able to increase any customer’s allocation in the near future.”
As of July 10, Puerto Rico had done 284,715 molecular tests and 228,665 serological or antibody tests, according to data from the Department of Health’s BioPortal platform. Private laboratories processed more than 85% of the total molecular tests done in Puerto Rico through the end of July. Although the number of tests has been increasing per month, they have fallen short given the demand.
To process tests in its own laboratories, the Department of Health relies on HHS, which supplies 100% of the reagents they use, along with swabs and viral transport equipment. That federal agency decides the quantities it will send to the island each week and at present, it does not deem Puerto Rico as a priority for the shipment of supplies.
“It has been requested, at the federal government level, that direct attention be given to those locations that have been identified in different states as having hotspots [geographic areas with a high incidence of the virus]. So different pharmaceutical companies are sending more reagents [through HHS] to those states that have serious problems, such as Florida and Texas. […] For the other states and territories, which are not in the same circumstances as those states that I just mentioned as hotspots, we’re receiving a smaller delivery of reagents,” said Mayra Toro, deputy secretary of the Puerto Rico Department of Health in charge of the Public Health Laboratories.
The lack of a robust system to carry out tests limits data on infection rates, reduces the ability to conduct contact tracing and hinders establishing preventive measures, according to an article written by specialists from the Puerto Rico Science, Technology and Research Trust, Harvard’s T.H. Chan School of Public Health and the Ponce Health Sciences University, and published in the American Journal of Public Health.
The lack of reagents to perform tests is happening at a time when Puerto Rico is reporting increases in confirmed deaths (85%) from COVID-19, compared to the previous month, while 392 people remained hospitalized as of August 17.
When the Vázquez administration assured that the epidemic was “under control” in April, May and June, the different phases of the economic reopening began, while lacking a clear strategy of to whom and when to administer the tests that were available.
In May, Department of Health Secretary Lorenzo González said there were enough tests and opened a call for doctors to make out referrals to perform them without restrictions.
“The floodgates opened, and thousands of additional tests began to be done daily,” said epidemiologist Melissa Marzán, who runs the surveillance system at Ponce Health Sciences University. “The reopening had an effect on the capacity to run molecular testing. And it seems that it was unplanned. Because if I open the economy and tell people that they need a [molecular] test to go back to work, we needed to know how many additional tests were needed for that phase.”
Leslie López, owner and administrator of the Laboratorio Clínico Román in the Arecibo urban center, confirmed how requests for tests skyrocketed in her business starting in June, with the reopening of the economy. “The demand is too much. It has been epic,” she said. “For now, we’re not testing, because I can’t do it and then send it to a reference laboratory that doesn’t have the reagents,” said López.
As of August 16, a total of 387,774 molecular tests had been done in Puerto Rico, according to data from the Department of Health’s website.
Government knew there would be a shortage of molecular tests
The Department of Health issued a guide on July 28 to define who would undergo molecular tests, during a spike in infections and after the lack of reagents turned into a crisis.
The guide prioritizes performing molecular tests on people with symptoms, hospitalized patients suspected of having the disease, Department of Health workers who manifest symptoms and provide hospital services, people who have been in contact with others who have tested positive, and pregnant women and children with symptoms of multisystem inflammatory syndrome, a generalized inflammation experienced by people who have been infected with COVID-19.
According to the version the Department of Health offered to the CPI, it wasn’t until June 19 during a meeting with laboratory representatives that it learned of the shortage of reagents for molecular tests in private laboratories on the island.
“There was no need to establish these guidelines [before the meeting] because we believed there were no restrictions or limitations on the availability of reagents at the private clinical laboratory level, as well as in the Department of Health,” said Mayra Toro. The guide arrived a month and a half after that meeting.
But the CPI validated that the government knew long before that reagents would be scarce on the island.
On March 20, there was a meeting in La Fortaleza between Gov. Vázquez Garced, former Department of Health Secretary, Concepción Quiñones de Longo, and members of the Association of Clinical Laboratories, as well as reference laboratories, and representatives of Ponce Health Sciences University.
Toledo, of the Toledo Clinical Laboratory, warned at the meeting that there was a problem with the availability of tests and said that that would be the case as long as the pandemic is ongoing. “It has been consistently said that there has been a shortage of reagents,” she confirmed.
Although the Secretary of Health admitted there was a decline in reagents, he insisted in a television interview on August 19th that tests are available and that he hopes to increase the volume that is processed by public laboratories. As for private facilities, González said he will ask them for daily updates about their inventories to steer people to those places where tests are available.
Faced with the shortage, HHS sent a notice on April 5 to public health laboratories in states and territories. It said that, in order to give public labs priority and provide them with reagents from a single source, the Centers for Disease Control and Prevention (CDC) would be buying these materials directly from private manufacturers and distributors. “[Public] laboratories are encouraged to order reagents in line with their immediate testing needs so that all states and territories are able to support ongoing testing while commercial manufacturers’ inventories are relatively constrained.”
The Department of Health focused on a strategy based on performing a greater number of serological or antibody tests, which are non diagnostic and have a high probability of giving false results.
In its plan unveiled in July, the government changed its strategy and now estimates that, together with private laboratories, 154,420 monthly molecular tests will be carried out between July and October, and up to 241,100 samples per month in November and December, which represents a monthly increase of 1,900% compared to the original plan.
Production on the island almost non-existent
On April 1, when the Department of Health was already headed by Secretary Lorenzo González, there was another meeting with him, the Governor, the private laboratories and members of the Medical Task Force in La Fortaleza.
Gynecologist Juan Luis Salgado, a member of the Medical Task Force, “talked about writing a letter to Roche to see if they could increase reagent supplies or shift their production line to Ponce. And he talked about the possibility of contacting Abbott to get a mechanism to do the rapid PCR process [a molecular technique for diagnosis]. The topic of reagents was an issue because Toledo Clinical Laboratory was the only one that was receiving them,” said Rexach and Toledo confirmed it.

Photo by Dennis A. Jones | Metro PR
The gynecologist Juan Salgado during the public hearings in El Capitolio about the purchase of tests.
However, Roche rejected the request to open a production line for the island. “Roche’s facilities in Puerto Rico are not intended for manufacturing molecular tests, which requires a highly specific type of production facility,” the company said in written statements to the CPI. The tests and reagents are produced in a “custom-built” factory in Branchburg, New Jersey.
The pharmaceutical industry, one of the pillars of the local economy, has almost 50 manufacturing plants in Puerto Rico. Today, only one of the companies, GK Pharmaceuticals in Manatí, is in the process of obtaining approval to produce reagents on the island, according to Deputy Assistant Secretary of the Department of Health, Mayra Toro. GK Pharmaceuticals obtained a pre-approval from the U.S. Food and Drug Administration (FDA) a few weeks ago and is currently looking into whether it can produce the reagents needed by laboratories in Puerto Rico.
Both the Department of Health and Puerto Rico’s reference labs use machines that they bought from Swiss company Roche Diagnostics for the Zika crisis in 2016.
They only allow working with a company’s branded reagent kits, as part of a methodology that is approved by the FDA.
Some labs have had access to reagents because they had machines from other companies, such as Hologic’s Robot Panther System, but this company also reported at the end of June that it had a limited supply of materials, Rexach added. Meanwhile, another company, Cepheid, developed a machine to do a rapid molecular test, but it has also been unable to keep up with demand.
According to Toro, the Department of Health has been on the waiting list for months for a Panther machine, as well as new equipment from Cepheid. The agency did not provide the dates when the process to acquire this equipment began.
The CPI asked Cepheid, Abbott and Qiagen, manufacturers of molecular tests similar to Roche, about the availability of their materials and the shipment date to Puerto Rico but got no response.
“Laboratories have never been in a position to get a surplus of reagents to stockpile. We’re living paycheck to paycheck. These companies are the ones that decide,” Rexach said. “Puerto Rico wasted the resource during the economic reopening. The few that exist are like gold, and you have to know who to give them to and when.”
Roche did not answer why when it sends reagents to Puerto Rico it prioritizes its delivery to the Toledo Clinical Laboratory, instead of distributing equal quantities to all private facilities so they can expedite the process. “If the responsibility can be shared with other reference laboratories, it would be an excellent initiative so that community laboratories have where to send the samples,” added Rexach.
Ilia Toledo said even when receiving reagents, the volume of samples is so high that her lab lacks the capacity to process the tests on time. She denied that Roche has a preference for her lab, claiming that it receives more quantities because it’s the largest lab in Puerto Rico and because it was the first to convey its interest in the tests to the pharmaceutical company.
100% dependent on the U.S. government
The situation that Puerto Rico is now experiencing is similar to that of Spain in March 2020, when, at the peak of daily cases, it faced a shortage to do tests. However, although it was unable to acquire all the materials it needed, the government reached an agreement with Spanish biotechnology companies to suspend exports and sell them all of the reagents. Spain was able to do this because it has local production.
There are also examples of states that have had initiatives to procure their supply of molecular tests.
In July, the administration of Governor Andrew M. Cuomo in New York granted incentives to 12 companies to produce materials that help fight the pandemic. Those state contributions include $750,000 to the Rheonix company, which would develop rapid, same-day molecular tests that have already received emergency approval from the FDA.
As a result of the reduction in the shipment of supplies from the federal government, the states of Maryland, Ohio, Louisiana, Massachusetts, Michigan, Virginia and North Carolina announced this month an alliance to acquire 3.5 million antigens tests, another alternative for virus detection. This type of test is more reliable than the antibody test when it comes to detecting the presence of the virus when taking the sample.
The CPI asked HHS for a list of supplies that the government of Puerto Rico has requested to carry out molecular tests, as well as the criteria to determine quantities and dates of shipments, but the agency did not respond.
The Department of Health responded that every week since March 13 it asks the HHS for the reagents it needs to process tests in its public laboratories, as well as swabs and viral transport materials.
With the supplies that HHS shipped between March and the first week of August, the Public Health Laboratories processed 40,275 molecular tests (or an average of 1,900 weekly samples), performed only in hospitals and to first responders, according to the Department of Health.
As of August 6, there were reagents available to run 21,000 tests in the Department of Health labs, which would supply about four weeks at the current rate. As of August 19, the figure is at 60,000, an increase of more than 185% in 10 days. The CPI asked the Department of Health how the number of molecular tests available in public laboratories tripled but got no response.
Health Secretary Lorenzo González was not available for an interview.

Photo by Nahira Montcourt | Center for Investigative Journalism
Lorenzo González said in May that there was sufficient tests.
In addition to what it receives from the federal government, the Department of Health purchased 150,000 molecular test kits from Quest Diagnostics between March and June. These kits, at $75 each, are processed in the company’s labs, including facilities outside Puerto Rico.
This has led to delays of up to 10 days for Quest to submit results, the Department of Health admitted. At best, the wait time ranges from three to four business days. The Department of Health’s contracts with Quest provide that the company would not be in breach for submitting late results.
As of August 19, the Department of Health had 45,146 Quest tests in inventory, according to the agency.
Beyond the Quest kits administered by the National Guard, the Department of Health has ruled out seeking additional supplies from the private sector that will allow, for example, to increase the inventory of reagents or swabs on the island.
Is there anything preventing the Department of Health from getting reagents, swabs, or viral transport outside of HHS?
“No, but at the same time, based on our responsibility, there hasn’t been a need for that,” Toro said. She argued that the Public Health Laboratories only process samples that come from medical institutions and have been able to meet this demand, so she said there is no need to seek out more materials.
But in May, Toro admitted to the CPI that the Public Health Laboratories were not operating at capacity. They were running about 250 tests daily out of the 600 they could analyze at the time, largely due to a shortage of swabs.
“In the beginning, there were no reagents to validate the equipment. That was remedied. Then the challenge was that we had swabs, but we didn’t have a way to transport them. At one point our laboratory had 6,000 swabs, but we had no means of transportation. We started to produce the means, but now there is a swabs shortage,” said the official at the time.
The agency distributes to hospitals the swabs and means of viral transportation that it receives from the federal government for sampling. The Department of Health then receives these samples for processing in its public laboratories.
The government, meanwhile, does not support private laboratories with supplies, despite the fact that they process eight times the number of tests in comparison to the Department of Health laboratories.
“The labs have asked the Governor for help. If [the government] provided financial assistance, I’m sure the labs would accept it,” said biochemist Marcos López of the Science, Technology and Research Trust. He mentioned as an example the Ponce Health Sciences University lab, which could automate its processes if it were to receive some type of subsidy.
So far, the government has awarded nearly $140 million to private hospitals and has more than $85 million in federal funds on the table for the acquisition of tests and tracking systems.
Fiscal Agency and Financial Advisory Authority Executive Director, Omar Marrero, was not available for an interview. The agency didn’t respond either if something prevented the government from earmarking part of these funds for private entities, as it did for hospitals.
A lack of will
López insisted that the Department of Health could “expand and set up its laboratories” to process molecular tests on the Island, but the agency never emerged as a key player in achieving this.
“They could have taken alternative measures [to increase their capacity], yes. But to say that the Department of Health is a key player to do tests in Puerto Rico, that’s not the case,” said López.
In the statement on the “negotiations with Roche,” Gov. Vázquez Garced referred to the pooling technique, through which several samples are analyzed in a group as if it were one, using fewer reagents in the process. If the test is negative, it means that the entire sample shares that result. If positive, the test is repeated individually for each of the patients. This methodology has been used in Germany, Chile, and places in the United States with positive results.
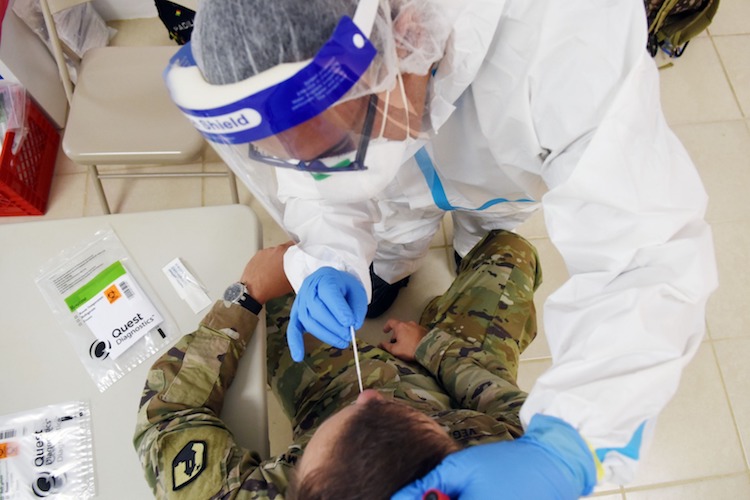
Photo by Dennis A. Jones | Metro PR
As of August 19, Salud had 45,146 Quest tests in inventory. The tests are administered by the National Guard.
The Department of Health has spent more than a month drafting “the methodology” to implement the process in the Public Health Laboratories, Toro said. However, the technique would not be as effective given the increase in the rate of positivity, or the number of positives among the total tests performed.
“If the positivity increases to 10%, the pooling is affected. Right now, it’s complicated [in Puerto Rico],” said Marcos López, who in April presented the idea of pooling to the government, along with a group of Puerto Rican scientists who at that time were seeking alternatives to the shortage. The positivity rate was around 4% then. Now it exceeds 16%, according to Department of Health data collected in COVID-19 Monitoring in Puerto Rico.
Marcos López assured that the scientific community expressed its support to the government’s response from the onset of the pandemic, including to process tests. Although he said he was pleased with the current relationship with the Department of Health, he lamented that it took so long to integrate the scientific community.
“It’s what we should have done from the beginning,” said the scientist.



